


How do you write the electron configuration for Carbon? The electronic configuration of Carbon will be 1s2 2s2 2p2. What is the electronic configuration of Carbon 6? What is the boiling Point of Carbon in Kelvin?īoiling Point of Carbon in Kelvin is 4300 K. Melting Point of Carbon in Kelvin is 3823 K. Using aufbau principle First, find electrons of antimony atom Periodic table. Each added electron occupies the subshell of lowest energy available. Both arrows in the 2p box should be pointing up. Electron configurations describe where electrons are located around the nucleus of an atom. What is the melting Point of Carbon in Kelvin? This procedure is called the Aufbau principle, from the German word Aufbau (to build up). What is the boiling Point of Carbon?īoiling Point of Carbon is 4300 K. Carbon has 6 electrons out of which 4 valence electrons are present in the 2s2 2p2 outer orbitals of atom. Principle energy levels are color coded, while sublevels are grouped together and each circle represents an orbital capable of holding two electrons. How many valence electrons does a Carbon atom have?Ĭarbon has 4 valence electrons. 5: Electrons are added to atomic orbitals in order from low energy (bottom of the graph) to high (top of the graph) according to the Aufbau principle. The element Carbon was discovered by Egyptians and Sumerians in year 3750 BCE. What is the color of Carbon?Ĭarbon is of Black color. It is located in group 14 and period 2 in the modern periodic table. Carbon is the 6 element on the periodic table. What is the position of Carbon in the Periodic Table?Ĭarbon is a chemical element with the symbol C and atomic number 6. Carbon is a chemical element with symbol C and atomic number 6. electrons in each orbital: STEP 1 Place the valence electrons in atomic orbitals using the Aufbau principle, the Pauli exclusion principle, and Hund's rule. To form abbreviated notation of electronic configuration, the completely filled subshells are replaced by the noble gas of the preceding period in square brackets. The abbreviated electronic configuration of Carbon is 2s2 2p2. What is the abbreviated electronic configuration of Carbon? The electronic configuration of Carbon is 1s2 2s2 2p2.
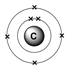
What is the electronic configuration of Carbon?


 0 kommentar(er)
0 kommentar(er)
